When you pick up a generic pill at the pharmacy, you expect it to work just like the brand-name version. But how does the FDA make sure it actually does? The answer lies in bioequivalence-a scientific standard that proves a generic drug delivers the same active ingredient to your body at the same rate and amount as the original. This isn’t guesswork. It’s a tightly regulated process backed by data, clinical studies, and strict thresholds that leave little room for error.
What Bioequivalence Really Means
Bioequivalence doesn’t mean two drugs look the same. It doesn’t even mean they contain exactly the same amount of active ingredient. It means that when you take them, your body absorbs and uses them in nearly identical ways. The U.S. Food and Drug Administration defines it as the absence of a significant difference in the rate and extent to which the active ingredient becomes available at the site of action.
Think of it like two different routes to the same destination. One might be a highway, the other a backroad. Bioequivalence ensures that both get you there at the same speed and with the same total distance traveled-no detours, no delays, no extra stops.
This standard was created under the Hatch-Waxman Act of 1984. Before that, generic manufacturers had to repeat all the expensive clinical trials done by the brand-name company. The law changed that. It allowed generics to prove equivalence through smaller, focused studies-saving time, money, and ultimately lowering drug costs for millions.
How Bioequivalence Is Measured
To prove bioequivalence, researchers run what’s called a crossover study. Usually, 24 to 36 healthy volunteers take both the brand-name drug and the generic version, in random order, with a washout period in between. Blood samples are taken at regular intervals over several hours to track how the drug moves through the body.
Two key numbers are measured:
- Cmax: The highest concentration of the drug in the blood. This tells you how fast the drug is absorbed.
- AUC: The total exposure over time-how much of the drug your body is exposed to overall. There are two types: AUC(0-t) for the period until the last measurable level, and AUC(0-∞) for the total exposure, including what’s extrapolated beyond the last sample.
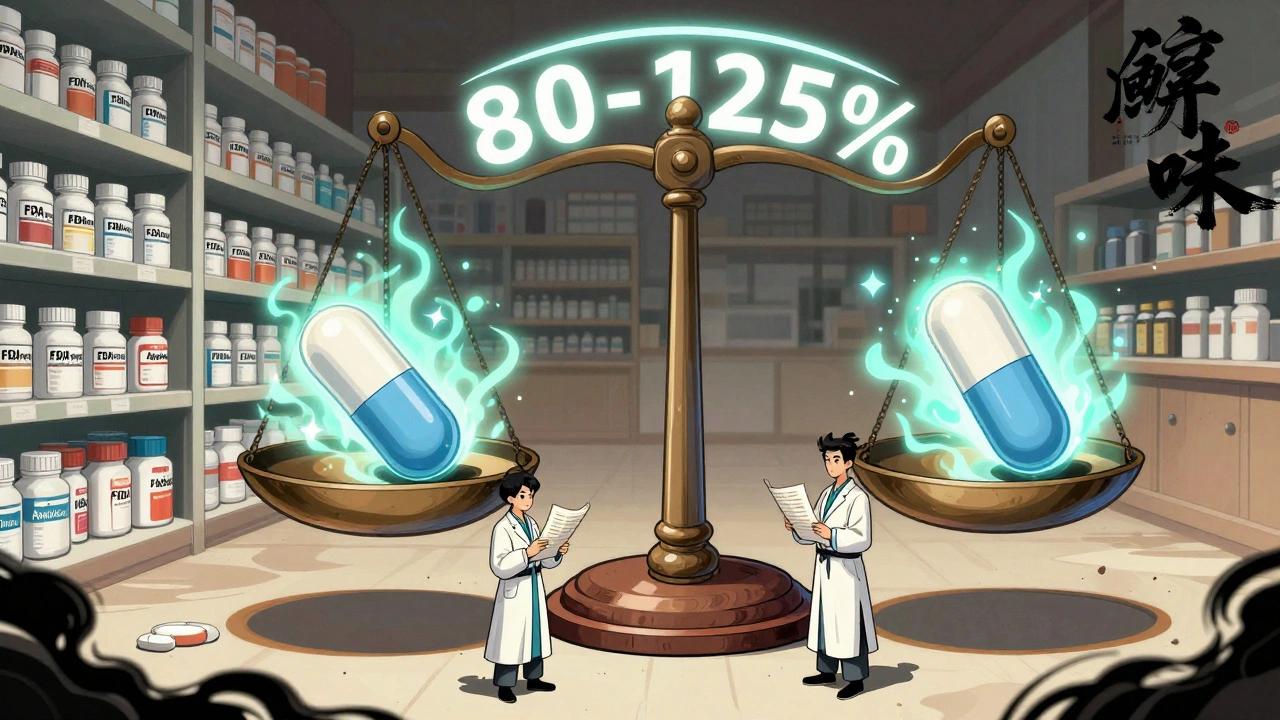
The FDA doesn’t just compare averages. It looks at the ratio of the generic’s values to the brand’s values. For both Cmax and AUC, the 90% confidence interval of that ratio must fall between 80% and 125%.
Here’s what that means in practice:
- If the brand drug’s average AUC is 100 units, the generic’s average must be between 80 and 125 units.
- But it’s not enough for the average to be within range. The entire 90% confidence interval must fit inside 80-125%. If the average is 93, but the confidence interval stretches from 75 to 110, it fails-even though the average looks fine.
- Conversely, if the average is 116% and the confidence interval is 103-130%, it fails because the upper limit (130%) exceeds 125%.
This isn’t a wide-open window. It’s a narrow, statistically sound range designed to catch even small differences that could affect safety or effectiveness.
Common Misconceptions About the 80-125% Rule
One of the biggest myths is that generic drugs can contain 80% to 125% of the active ingredient compared to the brand. That’s not true. The 80-125% range applies to how the drug behaves in your body-not how much is in the pill.
The actual amount of active ingredient in a generic must match the brand exactly. The FDA requires pharmaceutical equivalence: same drug, same dose, same form (tablet, capsule, etc.), same manufacturer standards. Bioequivalence is about what happens after you swallow it.
Even some healthcare professionals get this wrong. A 2015 study in PubMed found that a surprising number of clinicians believed generics could vary by up to 45% in active ingredient content. That’s not just inaccurate-it’s dangerous. The FDA’s system is built to prevent that kind of misunderstanding.
When the Standard Isn’t Enough
Not all drugs follow the same rules. For drugs that act locally-like inhalers, eye drops, or topical creams-the drug doesn’t need to enter the bloodstream to work. In those cases, the FDA allows in vitro testing: lab tests that measure how the drug dissolves or spreads.
But for drugs that need to reach the bloodstream-like blood pressure meds, antidepressants, or antibiotics-in vivo testing (studies in people) is required.
There’s another exception: drugs with a narrow therapeutic index (NTI). These are medications where even a tiny change in blood level can cause serious harm-think warfarin, lithium, or certain seizure drugs. For these, the FDA may apply stricter standards, though it still uses the 80-125% range as the baseline. Manufacturers of NTI drugs often need to provide additional data to prove consistency across batches.
What Happens Behind the Scenes
Applying for FDA approval isn’t just submitting one successful bioequivalence study. Since 2021, generic manufacturers must submit all studies they’ve conducted-even the ones that failed. This transparency helps the FDA spot patterns: Is the problem with the formulation? The manufacturing process? The excipients (inactive ingredients)?
Over 2,000 product-specific guidances are available from the FDA, each tailored to a particular drug. These documents tell manufacturers exactly what tests to run, what samples to take, and how to analyze the data. It’s like a recipe book for proving equivalence.
The ANDA (Abbreviated New Drug Application) review process typically takes 10 to 12 months. About 65% of applications get approved on the first try. The rest get deficiency letters-often because the bioequivalence data didn’t meet the 90% CI requirement, or the dissolution profile didn’t match the brand.
Why This Matters to You
Generic drugs make up about 90% of all prescriptions filled in the U.S. But they cost only about 20% of what brand-name drugs do. Over the past decade, generics saved the U.S. healthcare system nearly $1.7 trillion.
That’s not just a number. It’s a child getting their asthma inhaler. An elderly person taking their blood thinner. A parent choosing between groceries and medication. Bioequivalence standards make those choices possible without risking safety.
Every time you refill a generic prescription, you’re relying on hundreds of hours of lab work, dozens of volunteers, and a regulatory system designed to catch even the smallest deviation. It’s not perfect-but it’s rigorous. And it works.
What’s Next for Bioequivalence?
The FDA is exploring new ways to make bioequivalence testing faster and smarter. For complex products-like long-acting injectables, transdermal patches, or inhalers-traditional methods don’t always work. Researchers are now using computer modeling and simulation to predict how a drug will behave in the body, potentially reducing the need for human studies in some cases.
Industry experts predict a 20% annual growth in approvals for complex generics through 2025. That means more affordable options for hard-to-treat conditions. But it also means the FDA will need to keep refining its standards to match the science.
One thing won’t change: the commitment to safety. Whether it’s a simple tablet or a cutting-edge delivery system, if a drug wants to be called generic, it must prove it behaves like the original. No shortcuts. No exceptions. Just science.
What does bioequivalence mean for my generic medication?
It means your generic drug will work the same way as the brand-name version. The active ingredient enters your bloodstream at the same rate and to the same extent. You won’t notice a difference in how it works or how you feel. The FDA requires this proof before allowing any generic on the market.
Can a generic drug have different inactive ingredients?
Yes. Generic drugs can use different fillers, dyes, or preservatives than the brand-name version. These are called inactive ingredients, or excipients. But they can’t affect how the active ingredient is absorbed. If they did, the bioequivalence study would fail.
Why do some people say generics don’t work as well?
Sometimes, it’s not the drug-it’s the switch. If you’ve been on the same brand for years, switching to a generic can feel different, even if it’s scientifically identical. This is often psychological. But in rare cases, differences in how a drug is manufactured can cause minor variations in absorption. That’s why the FDA requires strict testing and why manufacturers must report all study results.
Are bioequivalence studies done on real patients?
Usually not. Most bioequivalence studies use healthy volunteers. This is because researchers need to control variables like diet, other medications, and health conditions. If the drug is meant for a specific population-like children or people with kidney disease-the FDA may require additional studies in those groups after approval.
How often does the FDA reject a generic drug for failing bioequivalence?
About 35% of generic applications get rejected on the first try, and bioequivalence issues are the most common reason. Many of these are fixable-manufacturers adjust the formulation, change the manufacturing process, or run another study. The FDA works with them to get it right.
Nancy M
December 3, 2025 AT 14:56Bioequivalence is one of those quiet miracles of modern medicine. You don’t see it, but it’s why your $4 prescription works just as well as the $40 brand. The 80-125% range isn’t arbitrary-it’s a statistical fortress built by pharmacologists who refuse to cut corners. I’ve worked in pharma compliance, and seeing those confidence intervals get scrutinized? It’s intense. But that’s what keeps people safe.
gladys morante
December 4, 2025 AT 23:03I’ve been on the same generic for years and never noticed a difference. But I’ve also seen people swear their blood pressure spiked after switching. It’s not always the drug-it’s the fear.
Precious Angel
December 5, 2025 AT 23:54Let’s be real-this whole bioequivalence thing is a government-sanctioned con. The FDA says ‘80-125%’ but what they don’t tell you is that some generics are made in factories where the air is thicker than the pills. I’ve seen the reports. Excipients? They use talc from questionable sources. And don’t get me started on the ‘healthy volunteers’-they’re paid college kids who don’t even know what they’re swallowing. This isn’t science-it’s corporate theater wrapped in a lab coat. And you? You’re the lab rat.
Every time you take that generic, you’re trusting a system that’s been lobbied into submission. The Hatch-Waxman Act? It was written by Big Pharma insiders pretending to help the poor. They didn’t want to spend money on trials-they wanted to monopolize the market under a different name. And now we’re told to be grateful? Grateful that our lithium levels might swing because someone in India skipped a QC step?
And don’t even mention NTI drugs. Warfarin? That’s a blood thinner. One percent off? You could bleed out. But the FDA still uses the same 80-125% rule? That’s not science-that’s negligence dressed up as regulation. They don’t test enough. They don’t follow up enough. And when the lawsuits start? They bury it under ‘post-market surveillance.’
Meanwhile, the brand-name companies? They just tweak the formulation slightly, slap on a new patent, and charge $200 again. It’s a cycle. And you? You’re just the sucker who keeps buying the same lie.
I’ve read the ANDA deficiency letters. Over half the time, they’re rejected for dissolution profiles. That’s the fancy word for ‘the pill doesn’t break down right.’ So they tweak it. Run another study. Submit again. And you think it’s safe? It’s not. It’s just statistically lucky.
And now they’re talking about computer modeling? That’s how you get another Vioxx. You don’t simulate a human body-you test it. Real people. Real conditions. Not lab rats on a budget. This isn’t progress. It’s a shortcut to disaster.
They say generics saved $1.7 trillion. Fine. But how many people died because the ‘equivalent’ pill didn’t? We don’t track that. We don’t want to know. And that’s the real scandal.
Melania Dellavega
December 6, 2025 AT 04:42There’s something deeply human about how we treat medicine. We want certainty-exact doses, identical results. But biology doesn’t work that way. Even two people taking the exact same brand-name drug can metabolize it differently. So why do we panic when a generic is 92% instead of 100%?
The 80-125% range isn’t a loophole. It’s an acknowledgment that human bodies are messy. It’s a tolerance built on decades of data, not guesswork. The real wonder isn’t that generics work-it’s that we’ve built a system that lets millions access life-saving drugs at 1/5 the cost, and still keeps them safe.
I’ve worked with patients who choose between insulin and rent. That’s not abstract. That’s Tuesday. And if a $4 pill keeps them alive, I don’t care if the filler is cornstarch instead of lactose. As long as the active ingredient behaves the same, it’s not a compromise-it’s justice.
Yes, there are bad actors. Yes, manufacturing varies. But the FDA’s transparency requirement-submitting every study, even the failed ones-is the antidote to corruption. That’s not bureaucracy. That’s accountability.
Maybe the real question isn’t whether generics work. It’s whether we’re willing to pay for the illusion of perfection. Because perfection is expensive. And sometimes, good enough is the most compassionate option we have.
Bethany Hosier
December 6, 2025 AT 21:08Have you ever wondered who owns the data from those bioequivalence studies? It’s not public. It’s held by the generic manufacturers. And the FDA only reviews it in private. What if the same lab runs all the tests? What if they’re owned by the same parent company as the brand? The system looks transparent-but it’s a closed loop. And we’re the ones swallowing the pills.
And why do they use healthy volunteers? Because sick people are too unpredictable. But what about the people who actually need the drug? The elderly? The chronically ill? They’re never tested. So how do we know it works for them? We don’t. We just assume. That’s not science. That’s hope.
And don’t get me started on the ‘2,000 product-specific guidances.’ That’s not guidance-that’s a maze designed to keep small manufacturers out. Only big corporations can afford to navigate that. So the market gets consolidated. Fewer choices. Higher prices. And we call it ‘affordable’?
It’s all a smokescreen. The FDA isn’t protecting us. It’s protecting the system. And the system? It’s designed to keep profits flowing, not people healthy.
Krys Freeman
December 8, 2025 AT 01:57Generic drugs are for losers who can’t afford the real thing. If you’re taking a pill made in China, don’t complain when it doesn’t work.
Shawna B
December 8, 2025 AT 05:37So the pill has to be 80-125% the same in the blood. But the actual drug amount is exact. So what’s the point of the 80-125% if the pill is the same?
Jerry Ray
December 9, 2025 AT 20:04Actually, the 80-125% isn’t about the pill-it’s about what your body does with it. The pill’s the same. But your gut, your liver, your metabolism? Those vary. So even if the pill’s perfect, your body might absorb it slower or faster. The 80-125% range accounts for that biological noise. If the generic’s absorption curve is too far off, it fails. It’s not about the tablet-it’s about the human.
And yes, some people feel different switching. Not because the drug is bad. But because their brain remembers the brand. Placebo effect works both ways.
David Ross
December 11, 2025 AT 16:47And yet, despite all the data, the science, the transparency, the FDA still approves generics that later get pulled for inconsistent dissolution profiles. The system isn’t flawless-it’s fragile. And we’re all just one bad batch away from a crisis. The 80-125% rule? It’s a band-aid on a bullet wound. We need real-time monitoring. We need blockchain-tracked batches. We need mandatory post-market pharmacovigilance. Not just ‘we’ll review it if someone dies.’ That’s not regulation. That’s gambling with lives.