When you pick up a generic drug at the pharmacy, you expect it to work just like the brand-name version. But here’s the thing: generic drugs aren’t tested on thousands of people before they hit the market. They’re approved based on studies with maybe 30 healthy volunteers. So how does the FDA make sure they’re safe once millions of people start taking them? The answer isn’t simple, and it’s not just about waiting for complaints.
What Happens After Approval?
The FDA doesn’t stop watching a drug the moment it gets approved. In fact, that’s when the real monitoring begins. Generic drugs are approved through the Abbreviated New Drug Application (ANDA) pathway, which means they don’t need to repeat the long, expensive clinical trials that brand-name drugs go through. Instead, they must prove they’re bioequivalent-meaning they deliver the same amount of active ingredient into the bloodstream at the same rate as the original drug. But bioequivalence doesn’t guarantee identical safety for everyone. That’s because the ingredients that don’t affect how the drug works-like fillers, dyes, or preservatives-can vary. And those differences? They can cause unexpected reactions in some people.For example, a generic version of a seizure medication might use a different binder than the brand name. For most people, it’s fine. But for someone with a rare allergy to that binder, it could trigger a serious reaction. The FDA knows this. That’s why they track what happens after approval with systems designed to catch signals no lab study ever could.
The FDA’s Surveillance Network
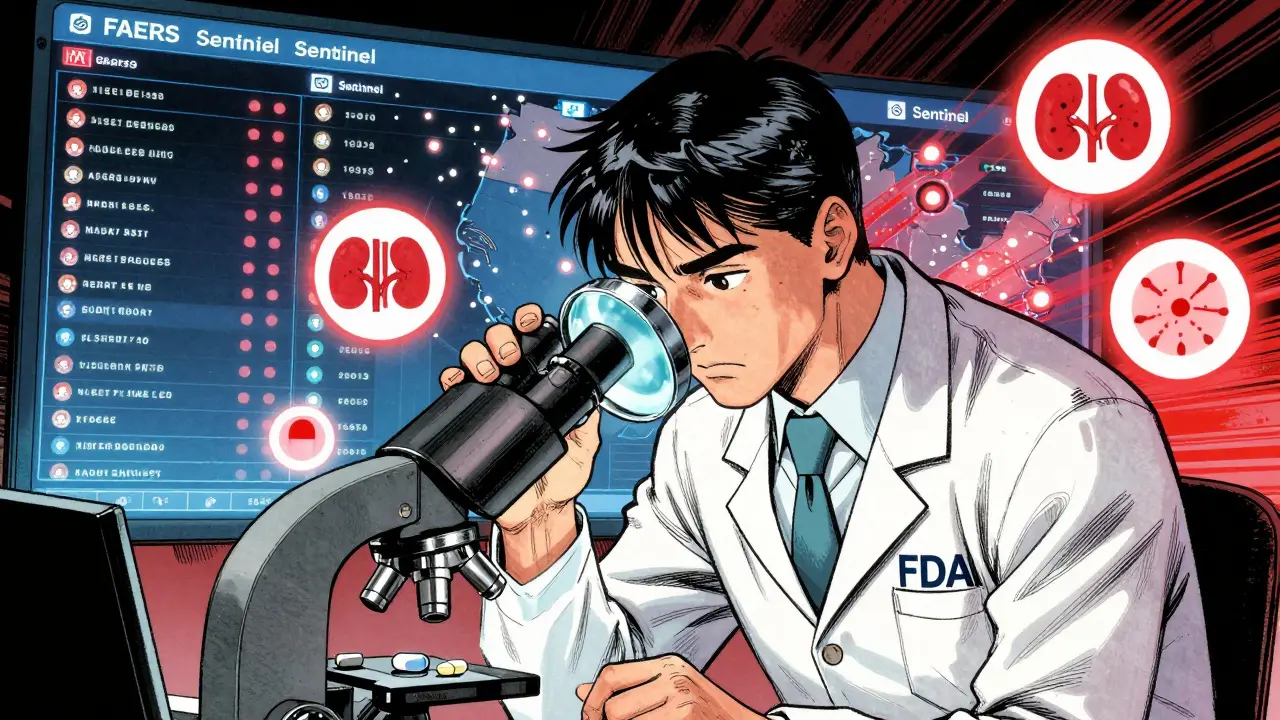
The FDA doesn’t rely on guesswork. They use a mix of automated systems, human analysis, and real-world data to watch for trouble. The backbone of this system is the FDA Adverse Event Reporting System (FAERS). Every year, FAERS collects around 2 million reports of side effects, hospitalizations, or deaths linked to medications. These reports come from doctors, pharmacists, patients, and drug manufacturers. When a pattern emerges-say, three different people report the same rare heart rhythm issue after taking a specific generic drug-the FDA digs in.But FAERS alone isn’t enough. Spontaneous reporting is messy. Studies show only 1% to 10% of adverse events are ever reported. So the FDA also uses the Sentinel Initiative, a real-time data network that pulls from electronic health records of over 100 million patients. Sentinel doesn’t wait for someone to file a report. It scans for patterns automatically. If a generic drug suddenly shows up in more emergency room visits for kidney issues than expected, Sentinel flags it. That’s how the FDA caught early signs of problems with certain generic versions of blood pressure meds in 2021.
Who’s Watching? The Teams Behind the Scenes
At the heart of this effort is the Office of Generic Drugs (OGD), part of the Center for Drug Evaluation and Research (CDER). OGD doesn’t work alone. It teams up with the Office of Pharmaceutical Quality (OPQ) and the Office of Surveillance and Epidemiology. Each group has a role:- OGD focuses on whether the drug still works like it should and whether its formulation changed in ways that could affect safety.
- OPQ inspects manufacturing sites-both in the U.S. and overseas. In 2022 alone, the FDA conducted 1,200 inspections at domestic plants and 600 abroad. They check for contamination, improper storage, or sloppy quality control. A single batch of bad pills can hurt thousands.
- Surveillance teams analyze data from FAERS, Sentinel, and even social media and medical literature. They look for clusters: Is this side effect popping up more often with this generic than the brand? Are certain patient groups-like older adults or people with kidney disease-being affected more?
These teams meet regularly. They don’t just react. They proactively screen certain generics, especially those with narrow therapeutic windows-drugs where a tiny difference in dose can cause toxicity or treatment failure. Think epilepsy, thyroid, or blood thinner generics.
Manufacturing Matters More Than You Think
One of the biggest myths about generics is that they’re all the same. They’re not. The active ingredient might be identical, but how it’s made? That’s where things get tricky. The FDA requires manufacturers to follow Current Good Manufacturing Practices (cGMP). But compliance isn’t always consistent. A plant in India might have a different humidity control system than one in New Jersey. That can change how the drug dissolves in your body. Even small changes in particle size or coating can alter absorption rates over time.The FDA uses risk-based inspections. If a company has had a past violation, they get inspected more often. If a generic drug is sold in high volume-like a popular statin or antibiotic-it gets more scrutiny. The FDA doesn’t wait for a crisis. They look at how many people are using it, how often it’s being reported in adverse events, and whether there are any changes in the manufacturing process. A single change in a supplier for an inactive ingredient can trigger a full review.
How the FDA Responds to Problems
When the FDA finds a safety issue, they don’t immediately pull the drug. They assess the risk. Here’s what they might do:- Update the label: Add a new warning about a side effect that wasn’t known before.
- Send a Dear Healthcare Provider letter: Alert doctors to watch for specific symptoms in patients taking the drug.
- Issue a recall: Pull a batch or even the entire product if contamination or potency issues are found.
- Require a Risk Evaluation and Mitigation Strategy (REMS): For high-risk generics, this might mean special training for pharmacists or mandatory patient monitoring.
In rare cases, if the risk outweighs the benefit, the FDA can request the manufacturer to withdraw the drug. This happened with a generic version of a diabetes drug in 2020 after multiple reports of severe hypoglycemia in elderly patients.

Why This System Works-And Where It Still Falls Short
The FDA’s system has come a long way since the 1990s. Back then, reporting was paper-based, inspections were rare, and data analysis was slow. Today, with Sentinel, FAERS, and GDUFA user fees (which brought in $65.7 million annually from manufacturers to fund monitoring), the system is far more responsive. The number of generic drugs approved each year has jumped to over 1,000, and the FDA is keeping pace.But challenges remain. Complex generics-like inhalers, injectables, or topical creams-are harder to monitor because bioequivalence is harder to prove. A generic inhaler might deliver the same dose, but if the spray pattern is off, the drug doesn’t reach the lungs the same way. These products don’t get the same level of post-market scrutiny as pills.
Also, underreporting is still a huge problem. Patients don’t always connect a new symptom to a drug they’ve been taking for months. Doctors are busy. They don’t always report. And when a generic is sold under multiple brand names, it’s harder to track which version caused the issue.
What You Can Do
You’re not powerless in this system. If you notice a new side effect after switching to a generic-especially if it’s different from what happened with the brand-name version-report it. Go to MedWatch or ask your pharmacist to file a report. The more reports, the better the data. Also, keep your medication list updated. If you switch generics, note the manufacturer. Some pharmacies will tell you which company made your pill. That info helps the FDA trace patterns.And remember: generics are safe for most people. They save billions in healthcare costs. But safety doesn’t end at approval. It’s a continuous conversation between regulators, manufacturers, doctors, and patients.
Are generic drugs as safe as brand-name drugs?
Yes, for the vast majority of people. The FDA requires generics to meet strict standards for bioequivalence, meaning they deliver the same active ingredient in the same way as the brand-name drug. Post-approval monitoring ensures that any unexpected safety issues are caught quickly. However, rare reactions can occur due to differences in inactive ingredients or manufacturing variations, which is why ongoing surveillance is critical.
How does the FDA know if a generic drug is causing side effects?
The FDA uses multiple systems. The FDA Adverse Event Reporting System (FAERS) collects reports from doctors, patients, and manufacturers. The Sentinel Initiative scans electronic health records from over 100 million patients to detect patterns automatically. The Office of Generic Drugs also proactively screens high-volume or high-risk generics for unusual trends in adverse events.
Can a generic drug be recalled after approval?
Yes. If the FDA finds contamination, incorrect dosage, or a safety risk that wasn’t apparent during approval, they can require a recall. This has happened multiple times-for example, when a generic blood pressure drug was found to contain a cancer-causing impurity in 2018. Recalls can be voluntary or mandatory, depending on the risk level.
Why do some people say generics don’t work the same as brand-name drugs?
This usually happens with drugs that have a narrow therapeutic index-like warfarin, levothyroxine, or seizure medications-where even small changes in blood levels can cause serious effects. Differences in inactive ingredients, manufacturing methods, or absorption rates can sometimes lead to subtle but meaningful variations. While the FDA requires bioequivalence, it doesn’t guarantee identical performance in every single patient. That’s why monitoring continues after approval.
How often does the FDA inspect generic drug factories?
The FDA inspects about 1,200 domestic and 600 foreign generic drug manufacturing sites each year. Inspections can be routine, scheduled, or unannounced. Facilities that have had past violations or produce high-risk drugs are inspected more frequently. The goal is to ensure compliance with Current Good Manufacturing Practices (cGMP) and prevent quality issues before they affect patients.
Jonathan Ruth
February 16, 2026 AT 07:12Philip Blankenship
February 17, 2026 AT 14:30Oliver Calvert
February 19, 2026 AT 06:16Kancharla Pavan
February 21, 2026 AT 02:20PRITAM BIJAPUR
February 22, 2026 AT 10:48Dennis Santarinala
February 23, 2026 AT 13:04Tony Shuman
February 25, 2026 AT 06:20Haley DeWitt
February 26, 2026 AT 07:28John Haberstroh
February 26, 2026 AT 18:18Geoff Forbes
February 28, 2026 AT 12:31Linda Franchock
March 1, 2026 AT 03:03Prateek Nalwaya
March 1, 2026 AT 03:46