NeoMeds - Page 2

How to Store Inhalers and Nebulizer Medications Safely: Temperature, Humidity, and Common Mistakes
Learn how to properly store inhalers and nebulizer medications to ensure they work when you need them most. Avoid common mistakes like bathroom storage, car heat, and humidity damage that can reduce effectiveness by up to 40%.

How to Report a Medication Error or Concern to Your Provider: A Clear Step-by-Step Guide
Learn how to recognize, document, and report a medication error to your provider or the FDA. Step-by-step guide to protect yourself and prevent future mistakes.

Generic Drug Labeling Requirements: What the FDA Mandates
The FDA requires generic drug labels to be identical to brand-name drugs, with only minor exceptions. This ensures patient safety but creates delays in updating critical warnings. Learn what’s mandated, how manufacturers comply, and why it matters.

How to Avoid Transcription Errors from E-Prescribing Systems
E-prescribing reduced handwriting errors but introduced new transcription mistakes. Learn how to fix misformatted dosing, duplicate prescriptions, and system gaps that still put patients at risk.

Diabetes Medications During Pregnancy: Insulin vs. Oral Options Explained
Learn which diabetes medications are safe during pregnancy, including insulin and metformin, and why other oral drugs are not recommended. Get clear, evidence-based guidance for managing blood sugar before, during, and after pregnancy.

Hormone Replacement Therapy: Benefits, Risks, and Monitoring in 2026
Hormone Replacement Therapy can effectively relieve menopause symptoms like hot flashes and prevent bone loss-but only when started early and monitored carefully. Learn the real benefits, risks, and how to stay safe in 2026.

Thyroid Medication Timing: How to Take Levothyroxine for Best Absorption
Learn the science-backed timing rules for taking levothyroxine to maximize absorption, avoid common mistakes with food and coffee, and understand why bedtime dosing might work better than morning for some people.

Health Literacy and Generics: Making Medication Information Clear and Usable
Many patients are confused when their generic medication changes appearance, leading to skipped doses and dangerous errors. Learn why this happens and what you, your doctor, and the system can do to make generic meds easier to understand.

OTC Medication Interactions with Prescription Drugs: What to Check Before You Take Them
Many people don't realize OTC meds like ibuprofen, acetaminophen, or antacids can dangerously interact with prescription drugs. Learn the top 5 risky combinations, who's most at risk, and how to check before you take anything.

Medication Errors in Hospitals vs. Retail Pharmacies: What You Need to Know
Medication errors are common in both hospitals and retail pharmacies, but the risks and causes differ. Learn how often mistakes happen, why they occur, and how to protect yourself from dangerous drug errors.
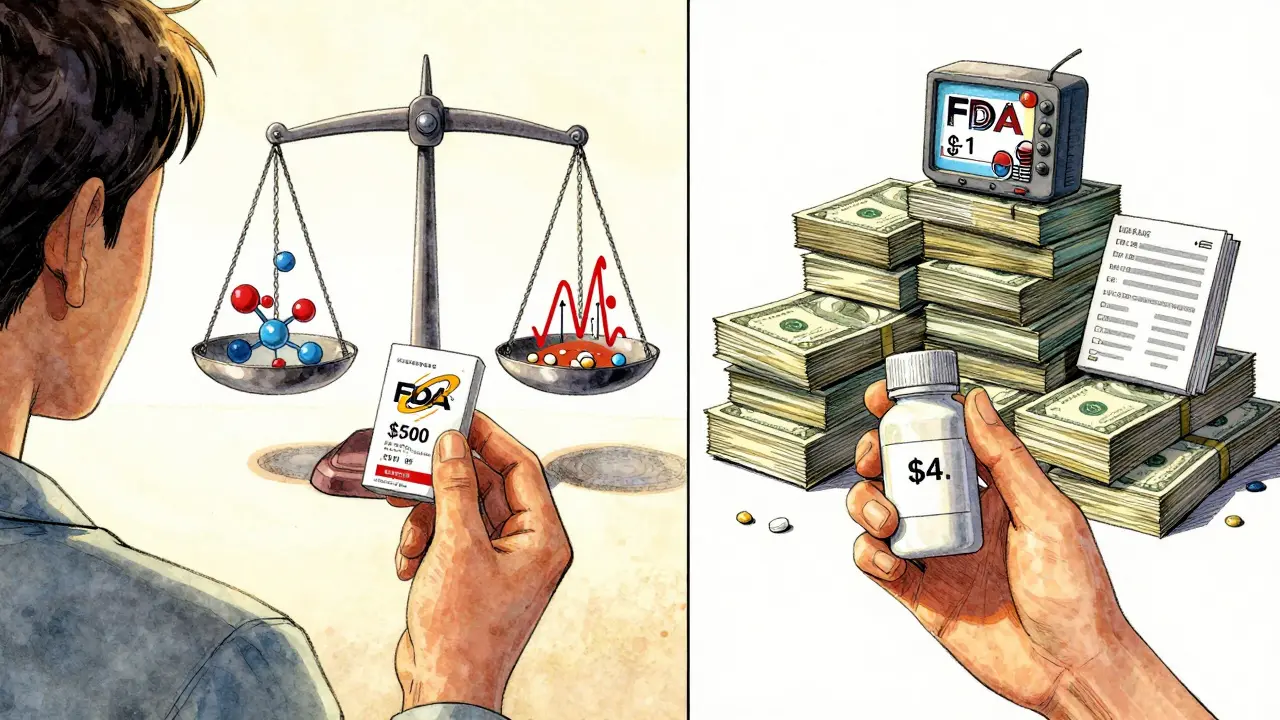
Why Generic Drugs Cost 80-85% Less Than Brand-Name Drugs
Generic drugs cost 80-85% less than brand-name drugs because they don’t repeat expensive clinical trials or pay for marketing. They contain the same active ingredients and meet the same safety standards - making them just as effective, but far more affordable.

Palliative and Hospice Care: How to Balance Symptom Relief with Minimal Side Effects
Palliative and hospice care focus on comfort, not cure. Learn how to balance effective symptom relief with minimizing dangerous side effects like over-sedation, confusion, and breathing problems.